Background:
Central venous catheters (CVCs) are commonly used in cancer patients to facilitate blood draws, administration of chemotherapy or other supportive care treatment. Catheter-related upper extremity deep vein thrombosis (DVT) is a major complication of CVCs, with studies reporting a rate of symptomatic venous thromboembolism (VTE) of 7% over 3 months in patients with active cancer and a new CVC. Despite the frequent occurrence, the optimal management in patients with cancer and catheter-related upper extremity DVT is unclear, given the lack of high-quality data.
Objective:
We conducted a survey aiming to characterize practice patterns and perceptions of clinicians who treat patients with cancer and catheter-related upper extremity DVT.
Methods:
An online survey was distributed to international clinicians who manage patients with cancer and catheter-related upper extremity DVT. The survey explored the type and duration of anticoagulation, focusing on the management after the initial 3 months of anticoagulation, as well as treatment strategies upon catheter removal and interests in future clinical studies. Descriptive statistics was used to summarize survey responses.
Results:
The survey was generated on LimeSurvey ® in English and publicized by Twitter ® and email communications via multiple international organizations of hematologists, oncologists, and/or thrombosis specialists, including the International Society on Thrombosis and Haemostasis (ISTH), Thrombosis Canada, the Canadian Venous Thromboembolism Research Network (CanVECTOR), and more. As of July 30, 2023, 115 clinicians from 26 countries had completed the survey. The majority of the respondents specialized in thrombosis (N=72, 62.6%) and/or hematology (N=60, 52.2%), followed by internal medicine (N=38, 33.0%) and oncology (N=14, 12.2%) (multiple selection was permitted for this question).
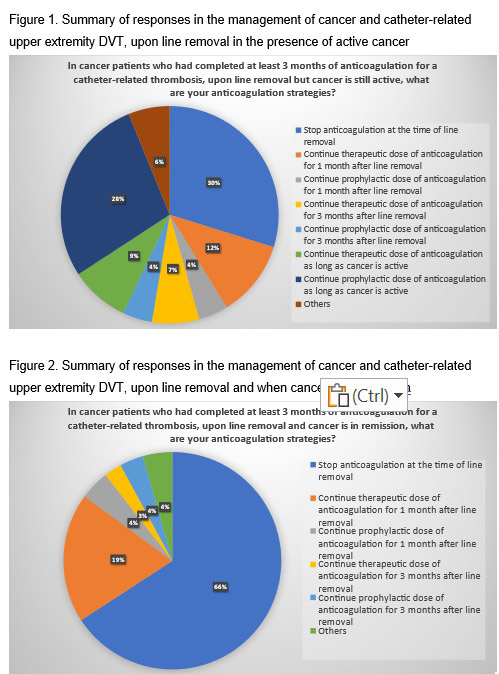
For the initial management of a patient without unresected gastrointestinal (GI) or genitourinary (GU) cancer, thrombocytopenia, or relevant drug-drug interactions, 75% of clinicians would prescribe a direct oral anticoagulant (DOAC) and 25% would give low-molecular-weight heparin (LMWH). On the other hand, in patients with unresected GI/GU cancer, the majority (N=105, 91.3%) of respondents chose to initiate LMWH. After the initial 3 months of anticoagulation, if the CVC remains in place in a patient with active cancer, the majority (N=112/114, 98.2%) of clinicians would continue anticoagulation, but with variable doses (57% and 43% chose therapeutic and prophylactic dosing, respectively). If the CVC remains in place but the cancer is in remission, fewer (70%) respondents would continue anticoagulation, and more would continue prophylactic as compared to therapeutic dosing (73% vs. 27%) in this setting. Practice variation further increases upon catheter removal, especially in the presence of active cancer (Figures 1 and 2).
The majority of respondents (N=111, 96.5%) agree that there is a need for prospective studies to evaluate the management of patients with cancer and catheter-related upper extremity DVT, and over 91% (N=105) would consider enrolling their patients in a study to evaluate prophylactic dosing of anticoagulation for secondary prevention of VTE in patients with cancer and catheter-related upper extremity DVT. To help plan such a study, we evaluated respondents' perception on the maximal “tolerable” recurrent VTE rate between 3 and 6 months (as the main interest is in the management strategy after the initial 3 months), and 2% and 3% were the most common responses (41.2% and 31.6% of the respondents, respectively).
Conclusions:
Our survey demonstrates that current practice in managing patients with cancer and catheter-related upper extremity DVT is heterogenous, especially after the initial 3 months of anticoagulation and upon catheter removal. Well-conducted prospective studies in this population are needed, which is supported by most clinicians.
Disclosures
Wang:Leo Pharma: Research Funding; Valeo: Honoraria. Delluc:Leo Pharma: Honoraria, Research Funding; Servier: Honoraria; BMS: Honoraria; Pfizer: Honoraria, Research Funding. Carrier:Bayer: Honoraria; Sanofi: Honoraria; Servier: Honoraria; Leo Pharma: Honoraria, Research Funding; BMS-Pfizer: Honoraria, Research Funding.